One of the exciting recent developments in our lab has been the identification of a previously unknown way in which the expression of a family of proteins are controlled. We have discovered a new “switch” that appears to play an important role in deciding which protein is made and, importantly, our data indicates that the control of this switch is lost in head and neck squamous cell carcinoma. This page will tell you a little bit about our ongoing research into this area. We will describe some of the initial findings that have set up the study and how we plan to use this information to try to either identify new ways to help diagnose the condition or, ideally, ways in which control of the defective switch can be restored as a new cancer therapy.
One of the exciting recent developments in our lab has been the identification of a previously unknown way in which the expression of a family of proteins are controlled. We have discovered a new “switch” that appears to play an important role in deciding which protein is made and, importantly, our data indicates that the control of this switch is lost in head and neck squamous cell carcinoma. This page will tell you a little bit about our ongoing research into this area. We will describe some of the initial findings that have set up the study and how we plan to use this information to try to either identify new ways to help diagnose the condition or, ideally, ways in which control of the defective switch can be restored as a new cancer therapy.
None of the work that is described here would be possible without the support of our funders. We are very grateful for the support from North West Cancer Research who support much of the work described below and we wouldn’t be where we are without previous support from the British Skin Foundation who funded a PhD studentship and American Cancer Society – Illinois, who supported me while I was at Northwestern University. NWCR and BSF are smaller than the likes of CRUK but they support some really exciting research, indeed they have greater flexibility to fund innovative projects. If you can, I thoroughly recommend supporting them.
We aimed to make this page accessible to all. If you would like more information or have other comments (eg if we haven’t explained something effectively), there is a contact box at the end, feel free to comment and we’ll get back to you and/or update the page!
One gene, many proteins
When you first study biology you learn that DNA is organised into genes and it is the genes that provide the code to make proteins. Good news, that is true! But, unsurprisingly, that’s not the whole story – one gene can actually make many different proteins. Indeed, this is really common; we now think that there are around 50,000 genes and there are probably more like 1 million proteins. The different proteins that are made from the same gene usually have quite similar functions but can be, and often are, different, and sometimes can even have the exact opposite function from each other! The switch that we are investigating is key to controlling which protein is made from a gene each time it gets activated.
The primary mechanism through which many proteins can get made from one gene is called “alternative splicing.” Splicing quite simply means joining together, in this case the joining together of the different parts of the gene that encode for protein. The alternative bit refers to the different ways in which these coding pieces can be organised.
We’ve made a short animation to explain some of the following points; the text description below the video explains everything a bit further.
Alternative splicing – a gene control mechanism
The simplest version of alternative splicing involves either using or not using a specific coding region (we call these coding regions exons). The one or more exons that get included or not included can code for different functional parts of the protein, so for example whether or not a specific exon is included could determine if a protein can act as an enzyme or not.
Whereas the “exons” are coding regions, the bits in between are called introns. These normally get removed from the final message that will be made into protein (called mRNA). However, in the type of splicing we are interested in this removal of the introns doesn’t happen as efficiently as it should so instead intron retention occurs. In normal human tissues intron retention doesn’t happen very frequently, or, if it does happen, it either is a short-term delay in splicing or the mRNA that gets made accidentally is recognised by the cell as junk and gets rapidly degraded. Notably however, in cancers there is growing evidence that intron retention rates increase dramatically.
In the splicing switch we are studying the intron retention event doesn’t lead to the mRNA being recognised as junk. Instead it gets treated as if were the real code for a specific protein. In this case, the specific intron that gets retained contains a code sequence that you would usually find at the end of a normal mRNA. This sequence gets recognised by the cellular machinery that is responsible for processing RNAs. These RNA binding proteins bind to this region and cut the mRNA at that point and then add a tail on the end that prevents the cut transcript from being destroyed. Indeed, adding this tail on the end means that the cells now recognise this intron-retained mRNA as being the proper code for a real protein and so this protein then is made.
So why is this a “switch”?

Ok, we have a situation where splicing can occur to make protein 1, and intron retention can lead to production of protein 2. Within any one cell there can many copies of mRNA at any one time for each protein type. When a gene gets turned on, some of the mRNA goes on to make protein 1 and some protein 2. Increasing the rate at which intron retention occurs means that not only is more protein 2 made, but also that less protein 1 gets made; the ratio shifts. Kind of like a bath or shower splitter system – turning the tap on provides layer one control, turn the switch to decide between bath and shower provides a second layer of control.
What does this mean for cancer?
So far we have avoided saying what the gene and proteins we are talking about are as we thought it important to get the key concept across first but now it’s time to get a bit more specific; the gene we are focused on is called LAMA3. This gene provides the code for three proteins, two large proteins – laminin α3a, laminin α3b, and an intron retained protein that we call LaNt α31.
LAMA3 has a little extra level of control in addition to that described above. LAMA3 has two promoters (the master on/off switches), one switch turns off laminin α3a and one turns on laminin α3b. The two large proteins that get made share about half their sequence but are very different in terms of size. They do similar things but in different ways and cells respond differently to them.
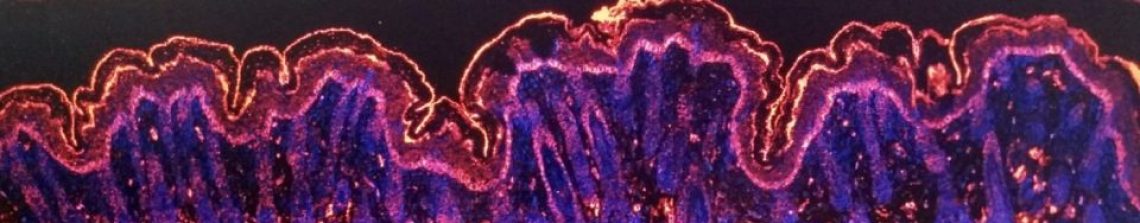
We’ll come back to laminin α3 function shortly, but now it’s time to see the switch in action… In the picture below the brown colour is where the proteins we are studying are found. In the top row we have normal tongue and we see lots of brown on the left picture (laminin α3b) and not much for the LaNt α31 on the right. In contrast, the bottom row picture come from the same person but at a site on their tongue where they have developed a squamous cell carcinoma. At this point, the laminin α3b on the left is almost completely absent and the LaNt α31 has been turned on. The switch has flipped.

What does flipping the switch mean for patients?
One of the primary goals of our current work is to find out the answer to this question! The data from the images above is very new, what we will do in the next few months is massively increase the numbers of patients we look at to see just how frequently we observe this phenomenon.
Importantly, what we will also do is combine those data with what we know about the patients’ outcomes to see if there is a clear trend. This could be really valuable information; for example if the people where the switch is activated are more likely to have metastasis then new patients could be screened in the same way, and if the switch is flipped in those patients then we would choose an aggressive treatment regime.
How is the switch controlled?
Although we know that the switch exists, we actually know very little about how it is controlled. Indeed, intron retention is the least studied form of alternative splicing and so not too much is even known on a more fundamental level.
Therefore the second aim of our current research is to identify how the splicing switch is controlled. With the information we get, we hope to be able to identify ways in which we can control the switch. We have a plan for how we should be able to do this but knowing more about how the switch works will let us target the right areas.
What do these proteins do?
Once either laminin α3a or laminin α3b get made, they form a complex with two other laminin chains (called β and ϒ chains), making a cross or t shaped larger molecule. These then get exported from the cell and end up being assembled into a structure that sheets of cells sit upon and use as their anchor points (you can sort of guess this from where the brown stain is in the top left corner in the pic above).
This structure, which we call a basement membrane, provides support for tissues in normal conditions, but also makes a huge difference in epithelial cancer progression. The basement membrane effectively acts as a barrier and as long as it remains intact the cancerous cells are restricted to growing in the epithelial layer i.e. they cannot invade and metastasise.
The Hamill lab actually does a lot of work studying laminins in different disease situations so, a couple of years ago, we made a short video introducing the laminins. We also wrote an intro page just talking about these amazing proteins, available here
 In terms of the intron retention product – LaNt α31 – we have an extra player. We named this protein “LaNt” rather than laminin α3c as the LaNt doesn’t contain the parts of the protein that really make laminins laminins, most notably the big structural domains that allow the laminins to be part of the basement membrane. However, the LaNt α31 protein does contain a so-called “laminin N terminal” domain, and experiments from our lab indicate that the LaNt protein can interact with laminins via this region.
In terms of the intron retention product – LaNt α31 – we have an extra player. We named this protein “LaNt” rather than laminin α3c as the LaNt doesn’t contain the parts of the protein that really make laminins laminins, most notably the big structural domains that allow the laminins to be part of the basement membrane. However, the LaNt α31 protein does contain a so-called “laminin N terminal” domain, and experiments from our lab indicate that the LaNt protein can interact with laminins via this region.
So, what does the LaNt interactions with laminins mean in terms of cancer progression? Well, right now, we don’t know for sure and some of the other people in the lab are doing experiments to try to find out the answer to this question! However, what we predict is that the LaNt binding to the laminins disrupts the network of laminins in the basement membrane and therefore weakens the barrier. We are testing this prediction with a series of experiments just now so will update this page as the data flows in. Until we have more data, we can’t be totally sure in the cancer situation as to whether it is actually the increased LaNt or the decreased laminin α3b that is the problem!
Conclusion
Thanks for reading this far! Hopefully this little taste has helped put our work into context and given you a flavour of what will happen next. In case you haven’t guessed, we love what we do in the lab! This research question has potential to lead to real world benefit to many patients and it’s really gratifying to feel that we could be on a path toward making a difference. It’s more than that though; it is really elegant biology and that makes it thoroughly interesting to find out more about! We couldn’t do anything without support from our funders, and so I would like to sign off by encouraging you again to check out North West Cancer Research and the British Skin Foundation or if you are in the US, the American Cancer Society – Illinois.
If you would prefer to support our research directly please get in touch, or you can donate via this paypal link.
Comments or suggestions are always very welcome