Materials and Methods or “Experimental Procedures” should be the easiest part to write of any scientific report, thesis, or dissertation – you know what you did! That said, in my experience of marking MRes reports and even doctoral theses, there are a lot of common mistakes that can be fixed and simple improvements that can be made to make this important section work more effectively.
Obviously, I am preaching to the converted as you have already clicked on this link! So, I am going to make this as painless as possible. I’ll highlight the important stuff with some examples and then you can bash out your methods sections with confidence.
Structure of this guide:
General comments and big tips first then links to a collection of specific details with examples of commonly used molecular biology techniques. Scroll to the end if you are looking to write a specific section (or via our quick tips page, here)
Big Tip #1 – Keep a good lab book!
This isn’t so much a tip but rather an absolute requirement for working in a lab!!
Usually the lab book is kept for the benefit of others and as a permanent record of your work. However, when it comes to writing methods, the lab book is key. Lack of details and you will struggle, especially if you ignore big tip #3!
PhD student Thanos wrote a blog post recently reviewing some of the electronic lab books that might help you improve your lab books. – available here.
Big tip #2 – Remember the key rules
- Write in prose, no bullet points!
- Write in the past tense throughout
- Write in the third person (don’t use I or we!)
- Use the correct form for units / measurements – (non italics, space between number and unit, no . after the unit. Not sure which units are correct: ukma-style-guide)
- Include all the details required to perform and interpret your experiment!
- Avoid repetition
- Reference where protocols came from
- Provide supplier information for all reagents
- Include full details of analytical techniques
- Define your statistical approaches
- Big tip #3 – Write your methods as close to the time you are doing your experiments as possible!
Two simple reasons for this
- You will find it easier as you will remember precisely what you have done and not have to solely rely on the quality of your lab book! Surely making things easier is reason enough!
- You need the details, all of them. If you have forgotten to record the batch or clone number of some key reagent it’s much easier to nip down to the fridge and find it than try and work it out later.
- Big tip #4: Use published work for examples – you don’t need to overthink this!
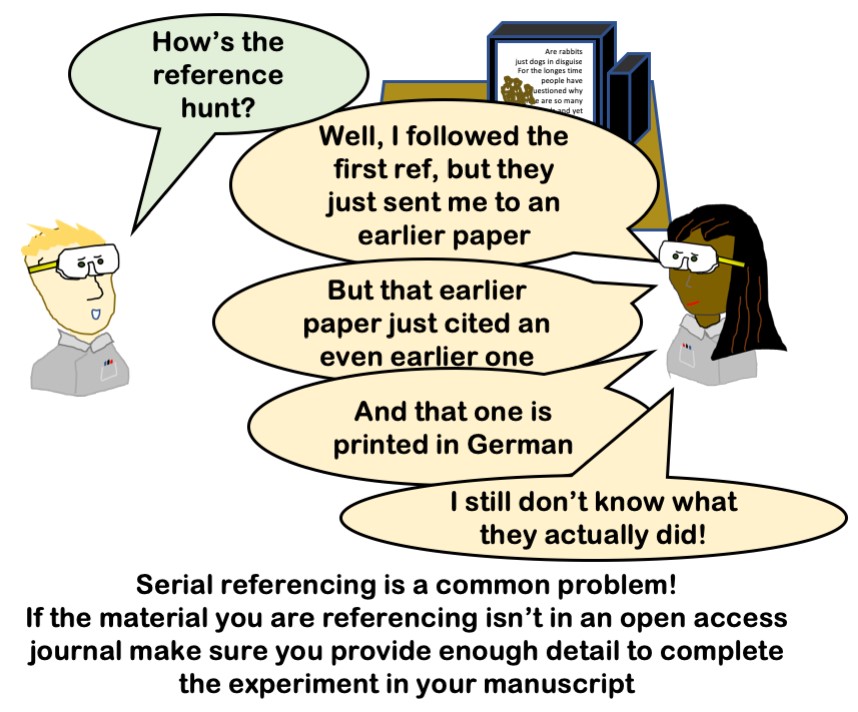
- Big tip #4.1: Cite well. Don’t send your readers down a rabbit hole
For most experiments, what you are doing is very similar or at least heavily based on something that has been published in the past. So start there! Use those publications to help you! This is especially true when it’s something that your supervisor has published before.
Provide the reference but don’t skip any of the experimental details that the reader would need to interpret the data. Don’t copy directly; carefully check all the details and change anything that needs changed.
As the next generation of writer, you should be doing things better than the generation before – so make sure you remove any ambiguities, tighten up phrasing add experimental specifics that were missed and generally do a better job!
If you are using a reference instead of writing out a part in full, make sure the paper you are referring to contains the whole methods and doesn’t send your reader down a rabbit hole of searching for data. In a thesis, you don’t have space limitations to the same extent so I would expect full details to be included and references there will cover where the original method came from and/or why you chose it.
- Big tip #5 Methods are not the same as a protocol
- Big tip #5.1 Understand the purpose of your materials and methods section and how it differs from results sections.
One of the most common mistakes I have encountered when marking projects is that there is a whole bunch of stuff that comes directly from the protocol but isn’t necessary to be defined in the methods. While the details are important, things like how the tubes were labelled, which well had which sample in it etc don’t need to be in there unless they actually impact the outcomes. Note that the order you processed the samples might be relevant (should be randomised and you should detail what method of randomisation your used!)
Standard techniques don’t need to be expanded. For example you can say “samples were centrifuged at 5000 x g for 5 min” rather than “samples were placed in a centrifuge and balanced then spun in the centrifuge for 5 mins at 5000 x g”
You shouldn’t be talking about data in your methods and your discussion of methods should be pretty minimal in your results (I use short “how” sentences/clauses to set up experiments but the main methods are in the methods! Results writing guide )
Sometimes, you might need to justify some of your decisions you made along the way but this is something that you should be cautious with and only do where it is truly necessary and beneficial. Some of these justification type observations are more powerful in your results section.
- Big tip #6: the details matter
This sounds like a contradiction to the protocols point but it’s not! The details of the experiment are the things that make the differences. At every step along the way you have made a decision on what to do/use, these decisions influence the data and they should be clear for all to see!
I’d like to emphasise something you many not have considered; you are not only writing this section so that other people can repeat your experiments but also you are writing so that academics can critically evaluate what you’ve done. Your readers/reviewers want to identify how robust your data is and where any limitations are so that they can come to their own conclusion about the data presented. They want to have enough information to decide if the claims you have made are appropriate. Importantly, this includes everything before data collection and everything after; knowing that your analysis was approached in the correct way, that you chose the right sample size and defined independence appropriately, and used the right statistical tests are all key to deciding if it was appropriate for you to come to the conclusions you did (don’t worry, I’ll cover stats below).
If you don’t write your methods effectively and comprehensively enough then your reader, reviewer or marker will have to make assumptions. Scientists are a critical bunch, if you leave them to make assumptions then they will assume the worst. I certainly do!
Just to hammer it home – in case you aren’t already convinced – think about when you come to defending your work during your thesis defence / PhD viva. Any place in your methods where you have been incomplete or ambiguous you are likely to be asked questions. Will you be able to remember the details of a series of experiments you did 2+ years ago whilst under the pressure of your exam?
- Big tip #7 – Follow a logical structure that will minimise the risk of repetition
Write the big, general stuff first
Start with the things that relate to multiple parts of your study. Things like antibodies, cell culture conditions, patient recruitment, mouse lines, construct generation etc. Get these down first and you’ll only need to mention them once.
Your goal is to deliver your methods in as clear and succinct a way as possible. Repetition is your enemy!
If you used the same plating or treatment strategy for your cells across multiple experiments then describe this once in a general section rather than repeat it. If you used multiple lines for the same experiment describe the consistent things once and highlight the specific details rather than writing out the whole experiment more than once.
As with other sections, it’s better to use forward references than backward – so make it clear when you first describe a set up that it will refer to multiple downstream applications rather than backward reference from the secondary experiments.
Regarding repetition. I once examined a PhD viva where the student had literally copied and pasted (with the same grammatical error) a two paragraph section 5 times in different places in their thesis. All within a few pages of each other. When I was reading it, I thought I was going mad. It was only when I asked him why he felt the need to say exactly the same thing 5 times that he realised how ridiculous that was. Don’t do this, nobody benefits!!
Next write the rest of your experiments!
I’m sure this surprised you! Seriously though, usually the best order is to arrange these next subsections in the order that you have decided to present the data. This isn’t a hard and fast rule, if you can make the methods shorter/easier to digest by doing it in a different order do that. But usually, it will help the reader if they know roughly where to look.
- Big tip #8 – Nail down the stats and analysis section
Right this is important! I’ve marked lots of MRes and undergraduate project reports and the data analysis and statistics section(s) of the methods are usually the areas with the biggest problems! I’ll deal with them separately:
Stats and analyses
How did you go from raw data to numbers? No use showing the reader a graph if they don’t know where the numbers came from!
For example, what did you use to normalise your data (dCt or ddCt? geometric mean? single reference transcript?). If you did experiments to justify your decision make sure to include that justification. How did you analyse your images? Include details on software used as well as the steps in post acquisition analysis.
For your stats, what you need depends on the type of your study. Here are some general tips:
- Include a clear description of the type of your study E.g. observational human study, animal experiment with full factorial design…
- Clear description of the variables measured – How you measured them, what their names are. In a human confirmatory study you need to say which variable is primary outcome.
- Clear description of statistical methods, How? This depends on the type of study you did but here are some general tips for statistical methods.
- Provide what simple descriptive methods you use (means and standard deviations for symmetric distributions, but medians, min and max for asymmetric distributions).
- Provide the names of all the statistical analysis methods used.
- Describe how you checked the assumptions of your tests (and what methods you used when the assumptions were not satisfied).
- If you use a more advanced stats method, provide reference to a book or a paper.
- Provide the level at which you defined as threshold for significance (p<0.05 etc. Also remember that p values above your threshold do not automatically imply that the null hypothesis is true, rather that you have insufficient evidence to reject)/
- Provide approaches you used to avoid bias: randomisation, blinding…
- Provide sample size statement – for confirmatory studies, i.e. studies with hypothesis.
Useful stats links:
- Experimental Animal studies – NC3R checklist. Tip; check your home office licence, power analyses and sample size calculations will have been defined for any hypothesis testing
- Observational studies – STROBE checklist
- Clinical trials – CONSORT checklist
Some journals require that the relevant guidelines/checklist be uploaded with your manuscript – check the instructions for authors – but irrespective if it is required or not you should still have a look as these are current best practice.
- Big tip #9 – Don’t go crazy with tables!
The general rules used by most journal is that “if you can deliver table contents in 4 lines of prose or less then you should write it out rather than use a table.” Simple as that. In a thesis you probably have a bit more flexibility but I would still follow the same general plan – unnecessary tables can be quite disruptive.
Usually you don’t use a table for a buffer mix or PCR conditions.
If you have loads of buffers that you want documented, you can use an appendix for that. Generally a ~6 component recipe fits perfectly well in brackets.
If you do use a table, make sure you format it appropriately and maximise it’s value by including all the details you need to deliver (eg for PCR primers include sequence, Tm, amplicon size and location not just the sequence).
Appendixes are more of a thesis thing. Consider for things that aren’t directly part of your key story. Things like cell line validation, buffer lists and product number lists or extensive data sets that should be included but would disrupt you results description.
- Big tip #10 – If you need figs, try to put them alongside the data rather than in the methods
This is bit more of a personal preference thing but I find that if you need a figure to explain your experiment then it works best directly alongside the actual data – usually adjacent to the primary data that came from the experiment.
You must describe your data figures in order of presentation so, in order to include a experimental schematic, you can work in a sentence to your results description in the “how” part of the appropriate results subsection.
Don’t use figures for standard set ups of common experiments (eg you don’t need the diagram of blotting apparatus from your protocol book.
Specific examples
I thought it would make sense to highlight some of the stuff and things that you should include and examples of how I have dealt with this in the past.
This page has got quite long so the links below will take you to a separate page about that technique/approach. I recommend bookmarking them so you can have them open as your write.