Laminin N-terminus alpha31 (LaNt α31) is a relatively unstudied protein that we began investigating recently and our recent work has just been published in Investigative Ophthalmology and Visual Science (IOVS). In this paper, we describe, for the first time, the distribution of this “new” protein across the surface of the eye in intact tissue and in times of active healing. Our studies have revealed some surprising findings that have implications for the understanding of how the cornea recovers from damage.
The full paper can be obtained from IOVS here (open access i.e. free). This page will describe some of the key findings our paper and is aimed at the non-specialist audience.
The front of the eye

Before we get going, let’s have some context. Our story focuses on the outside of the eye, the parts exposed to the wider world, the layers of cells that cover the surface (epithelium).
There are three main sections that are relevant: the cornea, the limbus and the conjunctiva:
- The Cornea. A transparent “window” in the centre of the eye, it’s through here that light passes and ultimately gets focused onto the retina. Because it needs to be clear, the cornea has few structural elements and does not contain blood vessels. The cornea provides most of the refractive power of the eye (the lens provides focusing)
- The Limbus (corneal limbus) is a relatively narrow ring of epithelium that encircles the cornea. This area is rich in blood vessels and contains populations of stem cells that supply the central cornea. Importantly for the work described here; when the cornea gets damaged the majority of the cells that are needed to replace the damage come from outside the cornea. If the limbus isn’t there, the conjunctiva grows over the cornea, a condition known as conjunctivalisation.
- The conjunctiva is the white part of the eye as you look at it. Cells within the conjunctival epithelium produce the tear film the covers the whole surface epithelium. Tear production relies on mucin producing cells (goblet cells) that are located there but not in the central cornea.
The extracellular environment a cell lives in is key to telling a cell what to be and controls activities. As each part of the surface epithelium carries out a different role, the cells of the epithelium produce different proteins and sit upon different extracellular environments. Studying a new protein in the front of the eye, and especially one that we think could be relevant to the function of different types of epithelium, was a logical place for us to start in order to get a first glimpse of function. A differences in distribution in the different epithelium could point us toward where to go next with our research.
Location specific differences in abundance and distribution

In the image above we have processed a human eye donated from for research using an approach called indirect immunofluorescence microscopy. This involves cutting very thin slices through the eye then probing those tissue slices using antibodies that we made that specifically bind to our protein of interest; LaNt α31 (introduction to these proteins via the link). We then probed the tissue slices with secondary antibodies that carry a fluorescent tag on them. These bind to places where the LaNt α31 antibodies are bound and using fluorescent imaging we can see where the secondary antibodies are bound. The camera on the microscope is black and white, hence the image above is black and white. However, we can use multiple different antibodies and different colours to look at more than one protein at a time and use false colouring to compare distributions.
In the image above we are looking at quite low magnification so we see all the different parts of the front of the eye. The brightness of the stain gives an indication of protein abundance (this technique isn’t great for abundance studies, so we are cautious with this interpretation), suggesting that the conjunctiva and limbal epithelium are relatively more abundant than the cornea. In the pics below, we have zoomed in to the limbus regions (yellow box above), and added an additional protein: LM332 which is a widely studied protein involved in basement membranes and contains a protein that is genetically related to LaNt α31 (simple introduction to laminins here or basement membranes here)

When we zoom in we see that the LaNt α31 staining is strongest in a line that separates the epithelial cells from the underlying matrix material, the bottom of the bottom (basal) cells of the limbal epithelium. Closer examination reveals that the line of staining is interrupted in places, including by the presence of melanin producing cells. When we compare the green (LaNt) stain with the purple (laminin) we notice that the green is actually just above the purple. We predict that these two proteins can interact with each other, the pics suggest that if that interaction happens, it is at the cell the boundaries.
Whenever you are studying something “new”, something that has not been investigated before, it is extra important to provide extra validation for everything. One approach is to study the same protein in different species. We therefore also made new antibodies that recognise the pig form of the LaNt protein and then processed pig eyes (obtained from an abattoir from animals used in meat production). The pics below are from the pig eyes and show a similar staining pattern. Subtle differences between pig and human sections could be the species specific or could be because the pig eyes are much younger (3 years vs 57 years in the human). Age-related changes happen a lot and can change the way the tissue function. It is something we would be interested in finding our more about for this protein in the future.


Fluorescent imaging is great for getting high-resolution images and directly comparing two or more proteins. However, you need fresh tissue to get robust, consistent results. As you can imagine, fresh eye tissue is very precious; most often the cornea gets used for transplants for people with burns or genetic diseases, so we turned to a different modality to expand on these studies.
A second option to get protein localisation data is to process tissues which have been embedded in paraffin wax shortly after retrieval and then locate the antibody binding using an enzymatic approach (immunohistochemistry). This approach not only allows us to use archived material that has been stored for many years, but also the paraffin helps to support the structures of the tissue e.g. stopping the epithelium from peeling off or getting ripped, and so using paraffin embedded tissue can complement the fluorescence imaging. The images aren’t as sharp but they provide a means to increase sample numbers and allow us to do some things that are more difficult without them.

The images above are from a human eyes processed with our two different types of anti-LaNt α31 antibodies and again you see the enrichment in the brown positive staining in the limbal region.
So, what’s going on in the limbus…. is LaNt α31 behaving like a stem cell protein?

The enrichment of LaNt staining in the limbus encouraged us to next compare the distribution relative to proteins known to be key to limbal function. including those that have been associated with stem cells and progenitor cells (image above). Although not identical, the patterns of LaNt α31, the transcription factor p63α, the cytoskeletal protein keratin 15 and the epidermal growth factor receptor are similar enough for us to next investigate what happens when stem cells become active.
In the images below, we have taken limbal tissue that was left over after corneal graft surgery and then grown the cells out from the limbal rim. In this situation, the stem cells in the limbus react as if a corneal wound has occurred and transition to a proliferative phase. In these conditions we see a general increase in LaNt α31 that correlates with increases in p63α, K15, EGFR in the same cell populations.

LaNt α31 location and abundance changes during corneal wound repair
One of the key functions of the limbal epithelium is to replace the cells of the central cornea following injury. The repair of corneal wounds involves cell division in the limbus and then the new epithelial cells migrate over the wound bed until the cornea is covered and the barrier is complete again. There are a lot of cell-matrix interaction involved in this process and, as we think LaNt α31 is involved in mediating these interactions, it seemed like a natural place to go next.
The images below come from a model of corneal wound repair that we have set up in the lab. Again, we’ve used pig eyes that would otherwise be discarded from abattoir to set up a model of a corneal burn injury. In essence, we use a culture system to keep the front part of the eyes alive. We then used an alkali soaked circle of filter to create a burn in the centre of the cornea and allowed it time to heal then processed the tissue for immunohistochemistry as before (images below).

What we saw was quite a dramatic change in LaNt α31 abundance and localisation during the different phases of wound repair. Essentially we see reduced/loss of expression as the wounds actively heal but then an increased staining along the base of the healed cell layer once the wound enters the maturation phase.
We also looked at the limbus to see what was happening there (below). As for our human explant, we see a big increase in expression but that increase is restricted to the middle stages of the repair process, it returns to normal once reepithalisation is complete and epithelium is maturing.

LaNt α31 – changing limbal epithelial cell behaviour
So, we have a protein that changes in expression and distribution but what does it do? Well we have been working on that too. There is a second paper in preparation that goes into a lot more detail, but for here we took human corneal epithelial cells and introduced the genetic elements to code for LaNt α31 using a virus. We also tagged the introduced protein with green fluorescent protein so we could track which cells were expressing it.
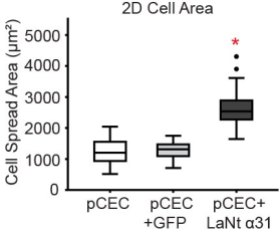
We then looked at two key cellular activities that are relevant to wound repair: spreading (an indirect measurement of cell-matrix adhesion) and migration. The graphs below show that cells that express a lot of LaNt α31 migrate less rapidly than control treated cells and display increased cell spread area. These findings connect will with what we have seen in terms of the changes in our wound model: expression of LaNt decreases as wounds heal but increases again when they need to be stuck down more tightly.
More to do
Together the findings we presented here indicate that this relatively unstudied protein could have an important role in corneal wound repair and possibly limbal stem cell behaviour. This really was a discovery science project, we don’t know about this protein and the more we know the better, but there are clinical implications of our findings. For example delayed wound healing is a feature of diabetic patients’ corneas, and there is a painful condition known as recurrent corneal erosions, where the healed epithelium isn’t firmly attached after wounds close. More information about what controls the processes will give us options for what to target or mimic in the future.
This paper is interesting on its own but really it marks the beginning of a story that we are working on. The next part is being prepared as I write this and will add a lot more depth and mechanistic understanding to some of these observations here. We also have a nice story coming together about what is controlling the changes we observe here and a bunch of ideas in development about how these findings can lead to therapeutic interventions in the longer term.
Funding
We couldn’t do research without the support of our funders. This work started thanks to support from Fight For Sight in the form of a New Lecturers’ grant when I first started my lab. Thereafter the two primary authors who did most of the work were supported by BBSRC (Valentina Barrera), and British Skin Foundation (Lee Troughton).
Want to know more?
Or interested in collaborating? Contact us via the form below.α