Aniridia is a rare genetic eye condition caused by changes in a gene called PAX6. This gene makes a protein (also called PAX6) that is an important player during eye development before birth, and then continues to support lifelong eye health. Indeed the PAX6 gene is often described as the master regulator of eye development. When one copy of this gene is not working properly, the body makes too little PAX6 protein, and this shortage affects many structures within the eye, most strikingly, reduction or absence of the iris at birth (The Greek work aniridia means “without an iris”).
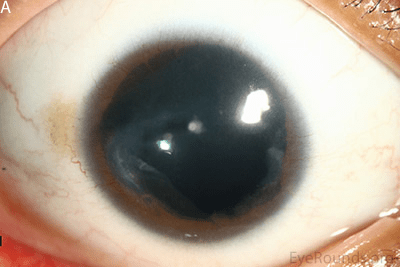
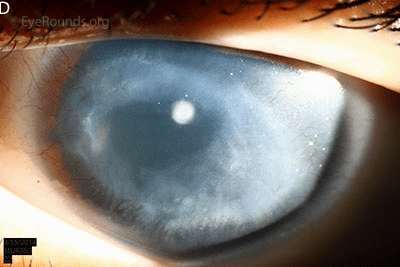
People with aniridia often experience other problems affecting their eyes including early onset glaucoma, and vision loss that slowly worsens over time. A particular problem is loss of function of the transparent front part of the eye, the cornea. This happens in later years as the stem cells that replace lost cells in the cornea slowly lose function over time. This corneal failure is painful and severely restricts vision. Current treatments focus on managing individual symptoms as they arise and lifestyle adjustments. Find out more, from a patient perspective here: https://aniridia.org.uk/
What is missing is a way to treat the underlying biological problem that causes these symptoms and that’s exactly the direction our project is trying to address.
Instead of Replacing the Gene, What If We Could Turn It Up? (image of dimmer switch)
In most cases of aniridia, one copy of the PAX6 works as normal whereas the other copy doesn’t make any protein. The functional copy is absolutely fine; it just cannot produce enough protein on its own. We call conditions like this “haploinsufficiency” which is just a fancy term to say that half is not enough.
We think that the presence of a functional genes means there is an opportunity:
If we could safely increase the activity of the working PAX6 gene, we might be able to restore the protein levels needed for a healthy eye. This idea, boosting gene expression rather than replacing the gene, is at the core of our new approach.
Working With MiNA Therapeutics to Develop Short Activating RNAs
Our lab is collaborating with MiNA Therapeutics (link), world-leaders in a technology known as short activating RNAs or saRNAs. Where many RNA therapies work by blocking harmful messages, saRNAs do the opposite. They are designed to increase activity specific genes, encouraging cells to produce more of a beneficial protein. The video below shows how they work.
The mechanism is a little complicated, but the concept is simple. In simple terms, saRNAs act like a gentle “volume dial”, helping cells turn up the gene that isn’t loud enough. So for aniridia, this means we want to identify saRNA that upregulates PAX6 and use it to boost expression of the needed protein.
One of the features of saRNAs that make them particularly exciting is that they don’t turn “on” genes that would otherwise be off, and this means that they will only be active in appropriate cells. This, in turn, means they will have much lower side effects than other ways of increasing gene expression. In addition, saRNAs don’t lead to massive “over” expression of the target gene; all the increases are within normal physiological limits. This, again, reduces the likelihood of side effects.
What We’ve Achieved So Far
Our work so far has focused on:
- Identifying precise regions of the PAX6 gene that saRNAs can target
- Collaborating with MiNA Therapeutics to design saRNAs optimised for strong activation
- Testing these molecules in cell models to see whether we can reliably boost PAX6 expression and “rescue” aniridia in cells in a dish.
The early signs are encouraging. We are seeing increases in PAX6 expression that suggest this approach could form the basis of a therapy in the future. Our next steps are working on delivering the saRNAs to the target cells and testing them in increasingly realistic pre-clinical models. This work is being supported by the eye research charity Fight For Sight who fund Dr Amyleigh Watts in our lab. Here she is scaling literal mountains rather than the mountains of PCR needed for this project.
What This Could Mean for Patients
Because aniridia affects eye development and ongoing eye health, even small increases in PAX6 could help stabilise or slow the progression of symptoms.
This approach cannot rebuild structures that are already damaged, and it will not be a cure. But it holds promise as a disease‑modifying therapy, one that could preserve vision for longer and potentially reduce the number or severity of complications over a lifetime.
For families affected by aniridia, the possibility of maintaining more stable vision and slowing the failure of the cornea — even modestly — is deeply meaningful. Aniridia might also just be the start; haploinsufficiency is the cause of about 1000 other inherited conditions, so if this approach works for PAX6 it could be adapted for some of these other conditions.
Next Steps in Our Research Journey
The steps ahead include:
- Refining the saRNAs to ensure they are effective, precise, and safe
- Conducting further laboratory work to understand how cells respond over longer periods
- Moving toward preclinical development in collaboration with MiNA Therapeutics
- Working with the wider aniridia community as we shape the future direction of the research
Therapeutic development is a long process, but each stage brings us closer to something that could genuinely change lives.
Staying Connected
We are committed to sharing progress openly as our work continues. If you live with aniridia, support someone who does, or simply want to follow our research, we invite you to follow the blog and get in touch. You can also check out Aniridia UK (link) as a patient support network.
This work is guided by science, but inspired by the people who may one day benefit from it.