We’re now over a week into our imaging sessions at HHMI Janelia using the iPALM microscope and it is time for a quick update. (part 1 here)
We’ve learned many things over the past week or so:
First, there’s no point having the best kit in the world if you don’t have people who can run it. The people working at the Advanced Imaging Centre are utterly fabulous. Indeed, whenever in future I say “we” did something on this trip, you can read that as either “someone from AIC did something” or (if it was quite simple) “we followed the carefully written instructions on how to do something”. Huge shout out to Anja, Hari, Leanna, Satya, Rachel, Leong and Jesse for all their help so far and thanks in advance for all their further help to come! Amazing scientists and lovely welcoming people.
Of that group m, special recognition to Dr Anja Schmidt. She is the one actually driving the iPALM and fighting daily with all the challenges this “diva” of a microscope throws up. We wouldn’t have any data if not for Anja et al.

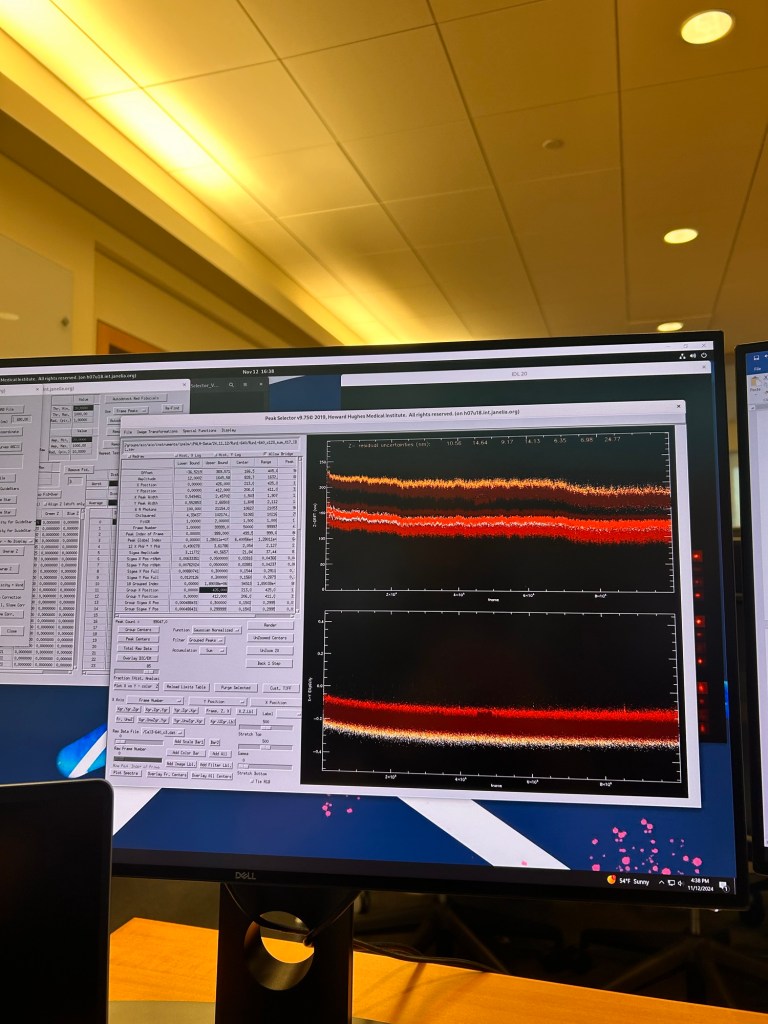
Second, millions of blinks acquired via 2 objectives into 3 cameras takes a lot of processing to convert into something useful. Thankfully Hari has written a 37 page document with images of what to expect and which of the several hundreds of buttons to press to adjust and correct issues such as drift in the XYZ planes. Running the macros takes hours and as each adjustment requires thousands of computations its quite slow work. I’m writing this whilst the computing cluster is processing the 100,000 x3 512×512 raw images from an imaging run. Below is a series of images from various steps along the processing pipeline to give you an idea of the interface… not trivial.









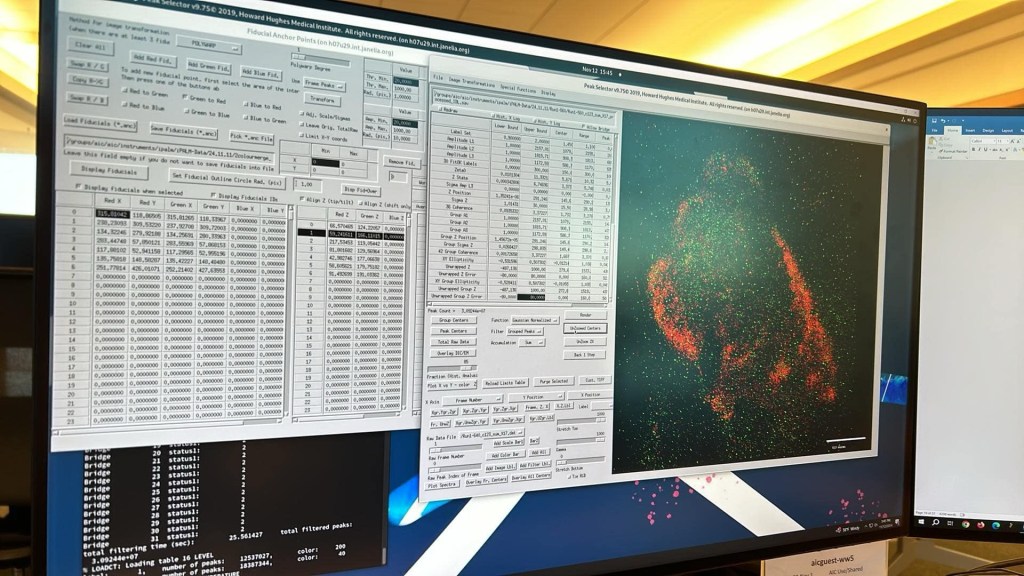
Third, fiducial (reference) beads are essential – density, location, brightness, they’ve got to be right. The coverslips we are using are covered in little beads that are bright and stable in the laser lines we are imaging. Or at least they should be. Last week we* (*Anja) encountered problems when the beads weren’t behaving properly. If you can’t find good beads, you can’t align the cameras. If you can’t align the ‘scope, you can’t image. If you don’t have beads in the correct place you can’t correct for drift or tilt and all your measurements will be off. Many of the processing steps and even choosing which cells to image in the first place depends on whether there are sufficient beads and how they are located relative to the point of interest.
Fourth, it’s good to have backup plans. We are onto plan B of our pre-trip plans (with plans C, D and E also tested and ready to enact if needed). Before we came over the fear of nothing working was very real. We did everything we could to test our constructs before getting here of course, but just because something works well on the fanciest of microscopes in Liverpool doesn’t mean it will work on the iPALM. Being able to pivot to a different way of answering our scientific questions has massively reduced stress and ensured we are able to spend the time processing images rather than changing hundreds of potential variables and living in panic.
OK, the macro has run. Time to get onto the next stage of processing. Look out for update 3 coming soon… hopefully containing images of fully processed cells. Here’s a wee tease… our first successful cell image with Anja (left), Natasha and Tom looking suitably happy.


2 Comments